Need a Zewski Report on your Device Idea?
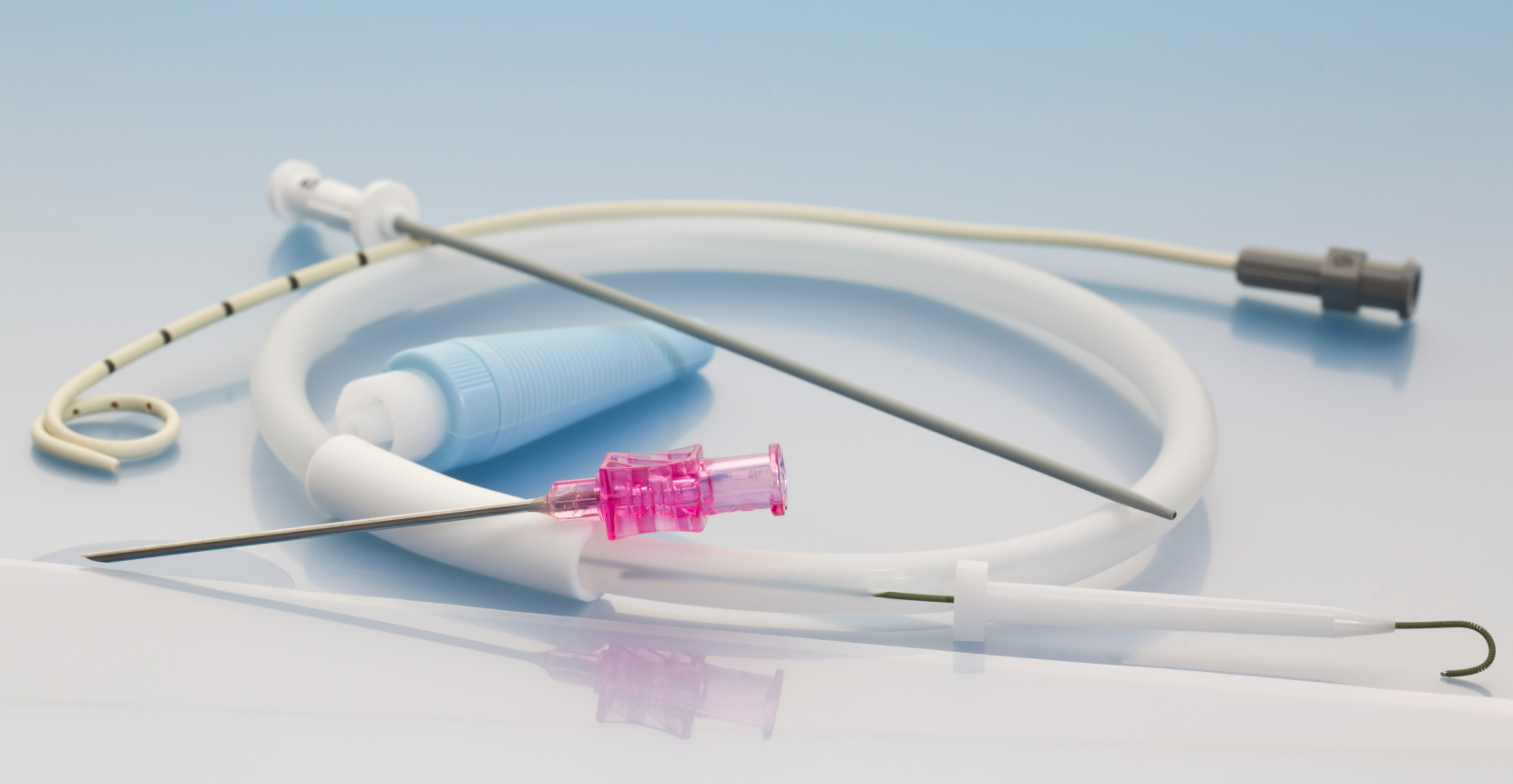
Percutaneous Catheter
ABOUT THIS REPORT
While this report focuses on the development of a percutaneous catheter, the insights provided are broadly applicable to similar medical devices. It's important to recognize that development timelines and costs can vary significantly between devices due to factors such as design complexity, regulatory requirements, and manufacturing processes. Nonetheless, the general principles and information outlined here offer valuable guidance for understanding the development landscape of comparable medical technologies.
FDA DESCRIPTION
A percutaneous catheter is a Class II medical device commonly used for therapeutic or diagnostic purposes. It is designed for minimally invasive access to the vascular or other bodily systems. The catheter is regulated under the FDA's Code of Federal Regulations (CFR) for medical devices. Most percutaneous catheters require FDA 510(k) clearance due to their intended use and potential risks, ensuring safety and effectiveness.
GENERAL DESCRIPTION
A percutaneous catheter is a thin, flexible medical device used for accessing blood vessels, delivering medications, or facilitating drainage during minimally invasive procedures. This device is an essential tool in interventional medicine, including cardiology, oncology, and nephrology. It is inserted through the skin (percutaneous entry) and guided to the desired location using advanced imaging techniques.
Percutaneous catheters are typically made from biocompatible materials such as medical-grade plastics and silicone, ensuring flexibility, durability, and patient safety. These materials are often combined with radiopaque markers to enhance visibility during imaging. The device may be used as part of a system, connecting to external components such as syringes, infusion pumps, or monitoring devices.
The design prioritizes sterility, ease of use, and compatibility with various clinical settings. Depending on its intended use, the catheter may include additional features such as side ports, specialized coatings to reduce friction, or integrated guidewires for enhanced maneuverability. Due to its versatility and critical role in patient care, the percutaneous catheter is a staple in modern medical practice.
PROJECT OVERVIEW
Understanding the general purpose of a percutaneous catheter is essential for informing its design and regulatory requirements. This device is intended for therapeutic applications, delivering targeted treatments to alleviate symptoms, manage conditions, or support recovery. It is designed to function as an accessory to a primary system, thereby enhancing overall functionality.
The catheter's manageable size strikes a balance between function and mobility, while its lightweight plastic construction makes it economical and relatively straightforward to manufacture. The inclusion of tubing components adds specialized functionality for fluid delivery, contributing to lower tooling costs.
Development will focus on addressing key clinical needs, with an emphasis on human factors engineering to ensure ease of use and patient comfort. Attention will also be directed towards cost-effectiveness, long-term reliability, and streamlined manufacturing processes to enable scalable production.
By adhering to these principles, the percutaneous catheter aims to provide effective therapeutic solutions while meeting both clinical and regulatory standards.
DEVELOPMENT COMPLEXITY
Developing a percutaneous catheter involves navigating various complexities to ensure the device meets clinical needs, regulatory standards, and patient safety requirements. The device's compact design enhances portability but necessitates precise manufacturing due to tighter tolerances. The use of medical-grade plastics, such as polyurethane or silicone, ensures flexibility and biocompatibility. Incorporating tubing components requires secure connections and durability under operational conditions, adding to the development and testing efforts.
 the "Project Management Triangle," also known as the "Triple Constraint" or "Iron Triangle."
the "Project Management Triangle," also known as the "Triple Constraint" or "Iron Triangle."
The absence of electronics and moving parts simplifies the development process. Without electronic components, there is no need for hardware or software testing, and the lack of moving parts reduces concerns related to mechanical failure or wear over time. Additionally, not requiring waterproofing or heat resistance allows for straightforward material selection and lowers production costs.
However, introducing novel materials 🏢 or coatings 🏢 can add complexity to the development process. For instance, hydrophilic coatings are applied to enhance the lubricity of catheters, facilitating smoother navigation through blood vessels. However, there have been instances where hydrophilic coating materials have embolized distally during percutaneous coronary interventions, leading to potential complications.
Therefore, any deviation from standard materials 🏢 or the introduction of new coatings 🏢 necessitates comprehensive testing to assess their impact on the device's performance, biocompatibility, and safety. Regulatory bodies may require extensive preclinical studies and clinical trials to evaluate these changes thoroughly. This ensures that the modified device meets all safety and efficacy standards before it can be approved for clinical use.
By focusing on these aspects, the development of the percutaneous catheter can achieve a balance between simplicity and functionality, ensuring both efficacy and safety in therapeutic applications.
🏢| Preferred vendors for this product service-specialty are available to registered users of The Zewski Report.
Cost & Time Estimates
TECHNOLOGICAL READINESS
Advancing the development of a percutaneous catheter necessitates a thorough understanding of its Technological Readiness Level (TRL), a framework that assesses the maturity of a technology from concept to deployment. Currently, if device is in the early concept development stage (TRL 2-3), where foundational ideas and preliminary designs are being explored.
Documentation and Regulatory Compliance
At this nascent stage, formal documentation may be minimal, facilitating rapid iteration. However, establishing comprehensive documentation early is crucial for maintaining consistent development practices, ensuring traceability, and preventing miscommunication or rework as the project progresses. Detailed records will be indispensable for future regulatory submissions, as medical devices must comply with stringent standards to ensure safety and efficacy. Adhering to these standards not only facilitates smoother regulatory approval but also enhances the device's credibility in the market.
Intellectual Property (IP) Considerations
The intellectual property position is promising, with a patent currently pending. Unique design elements, proprietary technology, or advanced methods of operation have been formally documented and submitted for review. The IP protection spans one country, offering security against potential competitors. As development progresses, it will be prudent to consider expanding IP protection to additional regions, especially in key markets, to safeguard the innovation and enhance the product's marketability.
Strategic Advancement
Recognizing the device's current TRL is vital for strategic planning. Each advancement in TRL signifies a reduction in development risk and a step closer to market readiness. By systematically progressing through the TRLs, the development team can identify potential challenges early, implement necessary mitigations, and ensure that the percutaneous catheter evolves from a conceptual design to a clinically viable and commercially successful product.
In summary, understanding and actively managing the technological readiness of the percutaneous catheter will provide a structured pathway for development, ensuring that each stage is completed with the rigor and diligence required for successful market entry.
REGULATORY APPROVAL
The percutaneous catheter is classified by the FDA under 21 CFR 870.1250 with the Product Code DQY, placing it within the Cardiovascular medical specialty. As a Class II device, it requires a 510(k) premarket notification submission to demonstrate substantial equivalence to a legally marketed predicate device. This process involves providing evidence that the device is as safe and effective as a similar device already on the market.
Reusable Design Considerations
Given that the catheter is intended for single-use applications, it is essential to ensure that the device is sterile and free from contaminants prior to each use. The FDA will closely evaluate the sterilization protocols to ensure they are effective and safe, as improper or incomplete sterilization could lead to contamination, infections, or compromised device performance. This single-use nature impacts several key areas of the regulatory pathway, including sterilization validation 🏢, biocompatibility testing 🏢, performance durability, and labeling.
Biocompatibility Testing
As the device involves percutaneous insertion, it is categorized as having "skin contact" and "tissue/bone/dentin contact" according to FDA guidelines. Therefore, the regulatory pathway will require focused biocompatibility testing to ensure that the materials used do not cause adverse reactions when in contact with the patient's skin and underlying tissues. This includes assessments for cytotoxicity, sensitization, and irritation or intracutaneous reactivity. The results of these tests will form a vital part of the submission to the FDA, ensuring that the device meets all necessary safety standards for its intended use. Additionally, careful material selection, labeling, and post-market monitoring are essential for achieving regulatory approval.
Therapeutic Application and Performance Validation
As a therapeutic device, the catheter will undergo moderate regulatory scrutiny, focusing on accurate and consistent therapy delivery, patient safety, and ease of use. Validation is required to prove its reliability under various conditions, including diverse patient populations, environmental factors, and usage scenarios. The regulatory process will involve comprehensive testing to demonstrate that it can safely provide effective therapy in diverse clinical settings.
In summary, while the 510(k) pathway offers a streamlined route to market, it demands thorough documentation and rigorous testing to ensure the percutaneous catheter's safety and effectiveness. Proactive engagement with regulatory requirements will facilitate a smoother approval process and successful market entry.
MARKET POTENTIAL
The device's design eliminates the need for modifications, making it highly adaptable to various healthcare settings and users. The lack of customization requirements reduces production costs and accelerates time-to-market, giving it a competitive edge. This simplicity also improves scalability. Uniform production processes can ramp up quickly to meet growing demand without the complexities of managing multiple designs. Its versatility ensures it meets various healthcare providers' needs, increasing its adoption potential.
Its functionality is similar to existing devices, offering little differentiation or competitive advantage in the market. Without unique features or improvements, it may struggle to stand out, limiting its market potential and making it harder to attract healthcare providers seeking innovative solutions or cost-saving alternatives.
🏢| Preferred vendors for this product service-specialty are available to registered users of The Zewski Report.
DEVELOPMENT PHASES & MILESTONES
Phase I | Concept Development (Inputs)
This phase centers on conceptualizing the device’s core functionalities and design. Key activities:
Market and User Research
Identify User Needs: Engage with healthcare professionals and patients to understand unmet clinical needs and usability preferences.
Assess Market Demand: Analyze epidemiological data and healthcare trends to estimate the potential demand for the device.
Product Comparison & Benchmarking: Examine existing devices’ features, performance, and user feedback to identify competitive advantages and areas for improvement.
Technical and Business Feasibility
Feasibility Study: Conduct both technical and financial assessments to determine the project’s viability.
Patent Landscape Analysis: Investigate existing patents to understand the intellectual property environment and avoid infringement risks.
Concept Development & Validation
Initial Ideation: Develop early concepts, sketches, and design outlines, focusing on key features and differentiators.
Concept Validation: Review the design with stakeholders (clinicians, engineers, regulatory experts) to gather feedback and refine the concept.
User Requirements Definition: Establish clear criteria for functionality, usability, and safety based on market and clinical input.
Regulatory and Compliance Considerations
Review Regulatory Requirements: Analyze the compliance landscape for similar devices to identify necessary safety and performance standards.
Incorporate Safety & Compliance Standards: Ensure early-stage design aligns with relevant regulatory guidelines to streamline future approvals.
Phase II | Prototype Development (Outputs)
This phase is dedicated to transforming the conceptual idea into a working prototype. The development will translate sketches into a physical model, addressing any technical challenges and laying the foundation for iterations. Key activities
:
Phase II Goals:
Prototype Build: Develop a functional prototype for the first round of internal testing.
Material and Component Selection: Finalize the materials and components used in the prototype, ensuring alignment with functional and regulatory requirements.
Refinement Cycles: Use early test data and feedback to modify the design and improve performance.
Design Reviews: Conduct periodic reviews with engineering and stakeholders to validate design progress.
Engineering Disciplines Involved:
🏢Human Factors Engineering: Ensure the design is user-friendly for healthcare professionals and safe for patients.
🏢Mechanical, Materials Engineering and Bioengineering: Ensure Design, Analysis & Fluid Dynamics are considered, focusing on the structural integrity and ergonomic design of the catheter, ensuring it meets clinical requirements. Analyze the flow characteristics within the catheter to ensure efficient delivery of therapeutic agents. Identify biocompatible materials that meet regulatory standards and are suitable for the catheter's intended use. Develop specialized coatings to enhance functionality, such as hydrophilic coatings for improved maneuverability.
🏢Clinical Integration: Align the device design with clinical workflows and requirements.
🏢Manufacturers: Selecting the right manufacturers is crucial for the successful production of the catheter. Manufacturers are often categorized into tiers based on their capabilities and roles within the supply chain.
Phase III | Testing (Verification and Validation)
This phase confirms that the device functions as expected.
-
Phase III Goals:
-
Performance and Safety Testing which validate the device's functionality in real-world operating conditions and conduct mechanical durability, biocompatibility, and electrical safety tests, where applicable.
- Failure Mode Analysis: Identify potential failure points and make necessary adjustments.
- Test Data Analysis: Review test data and document findings to support regulatory submissions.
The scope of testing expands to include clinical evaluation, assessing the device's performance and safety in a controlled clinical setting.
-
🏢 Biocompatibility Testing: To confirm that the materials used in the catheter are compatible with human tissue and do not induce adverse reactions, the following assessments are conducted:Cytotoxicity: Evaluates the potential of the device materials to cause cell damage.Sensitization: Assesses the likelihood of the device to cause allergic reactions.Irritation or Intracutaneous Reactivity: Determines if the device materials cause irritation when in contact with tissue.Hemocompatibility: Ensures that the device materials do not adversely affect blood components.🏢 Sterilization Validation: As the catheter is intended for single-use and must be sterile at the point of use, it's crucial to validate the sterilization process to ensure the elimination of microbial contaminants. This includes:
-
Sterilization Method Validation: Confirming that the chosen sterilization method (e.g., ethylene oxide, gamma irradiation) effectively sterilizes the device without compromising its integrity.Packaging Integrity Testing: Ensuring that the packaging maintains sterility until the point of use.🏢 Bench Performance Testing: These tests assess the mechanical and functional performance of the catheter under simulated conditions:Dimensional Verification: Ensuring the catheter's dimensions conform to design specifications.Tensile Strength: Assessing the force required to cause the catheter to fail under tension.Kink Resistance: Determining the catheter's ability to resist kinking, which could impede functionality.Torque Strength: Evaluating the catheter's ability to withstand torsional forces without damage.Leakage Testing: Ensuring there are no leaks when the catheter is subjected to pressure.Flow Rate Testing: Measuring the rate at which fluids can pass through the catheter.🏢 Coating Integrity Testing: If the catheter includes a lubricious or hydrophilic coating to enhance maneuverability, testing is necessary to ensure the coating's durability and uniformity:
-
Coating Adhesion: Assessing the bond strength between the coating and the catheter substrate.Particulate Evaluation: Ensuring that particles from the coating do not dislodge, which could pose risks to the patient.🏢 Shelf-Life and Packaging Testing: To determine the device's stability over time and the packaging's ability to maintain sterility:Accelerated Aging Studies: Simulating the aging process to predict the device's lifespan.Real-Time Aging Studies: Observing the device over its intended shelf life to confirm longevity.Package Integrity Testing: Assessing the packaging's ability to protect the device from environmental factors and maintain sterility.
FDA Guidance: Peripheral Percutaneous Transluminal Angioplasty (PTA) and Specialty Catheters - Premarket Notification (510(k)) Submissions Guidance for Industry and Food and Drug Administration Staff
Phase IV | Device Submission
This Class II device requires a 510(k) submission to demonstrate substantial equivalence to a legally marketed device (predicate). This phase involves compiling test data, including performance, safety, biocompatibility testing (where applicable), and preparing the 510(k) submission package. Collaboration with a regulatory consultant may be needed to ensure successful submission and timely approval. Main activities include:
Phase IV Goals:
-
Regulatory Filing Preparation: Prepare necessary documents and test data for submission to the FDA.
-
Risk Management File: Develop and submit a risk management file outlining potential risks and mitigation strategies.
-
Regulatory Feedback: Address any feedback or deficiencies identified during the review process.
-
Approval and Certification: Obtain regulatory approvals and certifications needed to market the device.
Feasibility
Understanding Your Feasibility Score
The Feasibility Score bar provides an assessment of your project’s path to market, with higher values indicating lower complexity and fewer anticipated obstacles.
- 0 - 39 (Low Feasibility): This range suggests that the project may face significant challenges due to high complexity or extensive requirements. Additional planning, resources, or risk mitigation strategies will be necessary.
- 40 - 74 (Moderate Feasibility): Projects within this range indicate a moderate path to market. While the overall complexity is manageable, some areas may require refinement or further development to ensure project stability and success.
- 75+ (High Feasibility): A score in this range indicates a relatively straightforward path to market, with low complexity and minimal additional work expected. This project is well-positioned to progress smoothly.
The Feasibility Score is a general guide, not an absolute measure of project success. We recommend using this score as part of a broader assessment and considering additional expert guidance for a comprehensive evaluation.
Cost & Time Breakdown
Phase I
Phase II
Phase III
Phase IV
Phase V
-
Cost and Time pertaining to vendor Tier point:
- Tier 1: Higher costs associated with comprehensive services complete system development, advanced technology, and the ability to manage complex projects. Design services may have shorter lead times due to ability to build a larger team however the scale of operations and the complexity of the more comprehensive supply chain may slow certain processes.
- Tier 2: Their cost and Time may vary based on their specialization allowing for efficient production of specific components, potentially leading to shorter lead times for those items. However, since they do not provide complete systems, the overall integration into larger assemblies may require additional coordination, potentially affecting timelines.
- Tier 3: Lower costs due to specialization in specific components or materials or limited staffing resources requiring additional coordination with other suppliers. This may slow the development time from both a design and supply chain perspective.
Considerations:
- Project Complexity: For complex projects requiring integrated solutions, Tier 1 suppliers may be more suitable despite higher costs and longer lead times.
- Cost Sensitivity: For projects with budget constraints, engaging multiple Tier 3 suppliers could be more cost-effective, though it may require more intensive project management.
- Supply Chain Management: Working with Tier 3 suppliers necessitates robust supply chain coordination to ensure timely delivery and quality assurance.
In conclusion, the choice between Tier 1 and Tier 3 suppliers involves a trade-off between cost, time, and the complexity of supply chain management. Careful evaluation of project requirements and resources is essential to make an informed decision.
-
Phase V | Device Production
Achieving production readiness for a percutaneous catheter involves meticulous planning, collaboration with specialized vendors, and investment in appropriate manufacturing equipment. The scale of production significantly influences both the choice of equipment and overall costs.
-
🏢Manufacturing Scale-Up: Ramp up production capabilities to meet market demand, ensuring quality control throughout the process.
-
Supply Chain Setup: Finalize contracts with suppliers and set up logistics for materials and components.
-
Quality Assurance: Implement quality control processes to maintain consistency and reliability during production.
-
Market Launch: Launch the device in the target market, supported by marketing and sales strategies.
-
-
🏢Original Equipment Manufacturers (OEMs): These vendors offer comprehensive services, including design, development, and manufacturing of medical devices. Partnering with an OEM can streamline the production process, ensuring adherence to industry standards and regulatory requirements.
-
🏢Contract Development and Manufacturing Organizations (CDMOs): CDMOs provide specialized services such as product development, prototyping, and large-scale manufacturing. They are particularly beneficial for companies seeking to outsource specific aspects of production to leverage specialized expertise.
-
🏢Testing and Validation Service Providers: Ensuring product safety and efficacy is paramount in medical device manufacturing. Vendors specializing in testing services offer comprehensive evaluations, including performance testing, biocompatibility assessments, and compliance verification with ISO standards.
-
- Essential Manufacturing Equipment:
-
🏢Injection and Extrusion Molding Machines: Utilized for producing precise plastic components of the catheter, ensuring consistency and high-quality output.
-
🏢Balloon Forming Machines: Essential for shaping and forming catheter balloons, these machines provide precise control over dimensions and material properties.
-
🏢Catheter Tipping Equipment: Employed to form the distal tip of the catheter, ensuring smoothness and precision critical for patient safety and comfort.
-
🏢Laser Cutting Machines: Used for creating intricate designs and features on the catheter, such as side holes or slots, with high precision.
-
🏢Automated Assembly Lines: Facilitate the efficient and consistent assembly of catheter components, enhancing production speed and reducing manual labor.
-
🏢| Preferred service providers who can assist in any of these areas of testing are available for this product service-specialty for registered user of The Zewski Report.
Disclaimers & Limitations
- Generalizations: This report provides a high-level overview based on standard assumptions and does not account for unique device characteristics. Actual costs, timelines, and risks may vary significantly depending on the device's design, use case, and target market.
- Assumptions of Device Class and Use: Assumptions were made regarding the device's classification and intended use. These assumptions can impact regulatory requirements, costs, and timelines. Specific regulatory pathways, for instance, may differ based on the device's risk classification and market entry strategy.
- Market and Regulatory Dynamics: Regulatory requirements and market conditions are subject to change. The report's cost and timeline estimates may be affected by evolving regulatory landscapes, standards, or unforeseen market dynamics, which could delay approval or require additional testing.
- Risk Assessment Limitations: Risk levels and mitigation strategies are based on general device categories and may not fully address specific technical or operational risks unique to the product. Thorough risk assessments should be tailored to the device's complexity, materials, and usage.
- Development Phases and Milestones: The development phases outlined here follow a typical medical device development pathway, but real-world project phases may overlap or require iteration due to unforeseen challenges or design changes.
- Cost and Timeline Variability: The cost and timeline estimates are based on standard industry benchmarks but do not account for project-specific adjustments. Factors like unexpected technical challenges, prototype iterations, or regulatory re-submissions can significantly impact final costs and schedules.
- Reliance on Industry Standards: The report relies on common industry standards for development and testing. However, additional standards specific to certain device features or regions may apply, affecting compliance requirements and associated timelines.
- Testing and Validation Scope: Testing and validation requirements are generalized. Devices with novel materials, complex electronics, or unique features may require additional, specialized tests, potentially extending both cost and duration.
.png?width=4200&height=441&name=Zewski_Report%20Logo%20(Shirts).png)