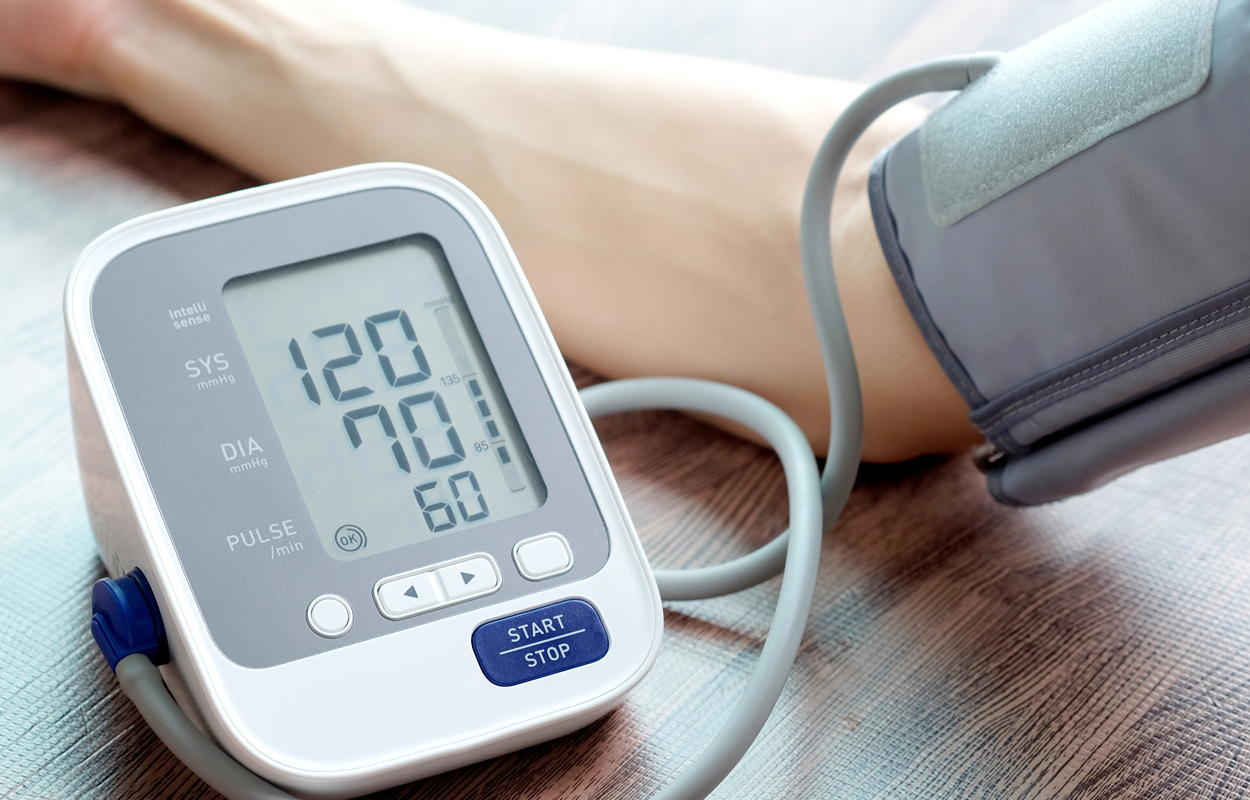
Blood Pressure Cuff
Device Type: Cardiovascular - Diagnostic
FDA Description:
A blood pressure cuff is a device that has an inflatable bladder in an inelastic sleeve (cuff) with a mechanism for inflating and deflating the bladder. The cuff is used in conjunction with another device to determine a subject's blood pressure.
To generate reports at The Zewski Report, we use a variety of sources for inputs. When analyzing an existing device, we will review the following items and create a Ballpark report on what we believe the development pathway would be in today's market for a device similar to the one known.
- FDA Records
- Recognized Standards
- Other Known Testing
- Review of Existing Design
- Review of Existing Assembly
In order to generate a report for a device that is not approved or designed, it takes a little more effort. However, we still can create a report with limited information. In this scenario, we will help identify your indications for use and review your patent. This allows us to home in on critical data including:
- FDA Records with Similar Intent
- Associated Recognized Standards
- Experience Based Test Knowledge
- Patent Review for Design Intent
- Known Technology Needed for Manufacturing
Device Classification
| Device Name | Blood Pressure Cuff |
| Regulation Description | Blood Pressure Cuff |
| Regulation Medical Specialty | Cardiology |
| Product Code | DXQ |
| Submission Type | 510(K) |
| Regulation Number | 870.1120 |
| Device Class | 2 |
| Implanted Device | No |
| Life Sustaining Device | No |
| Third Party Eligible | Yes |
FDA Approved Devices
Design Assumptions
The blood pressure cuff is a non-sterile, multi-patient use, reusable device and is designed to be used by a clinician to measure a patient’s blood pressure. It's typical duration of use is less than 1hr.
The device comprises an inflatable bladder that fills with air to apply pressure to the artery, an inflation bulb that is used to manually inflate the bladder, a valve to control the release of air from the bladder, and a tubing which connects the bladder to the bulb. The cuff is typically made of nylon with Velcro strips to allow the cuff to adhere to itself, so it remains in place during use. The blood pressure cuff is not exempt from good manufacturing practices, including design controls requiring design traceability documentation to be compliant with regulations.
Regulatory Assumptions
The blood pressure cuff is a moderate-risk Class II device categorized under regulation number 870.1120, which covers several product codes – DXQ being the focus of this report. The device is intended for facility use and has a 510(k) submission type and is eligible for third party review. It can also be noted that the FDA has fees for small businesses that may range between ~$8k (for a 510(k)) ~$35k (for De Novo) for adult indicated devices.
Testing Assumptions & Consensus Standards
The device is subject to various mechanical performance tests such as pressure, force, leak, kink, and drop tests. In addition to conditional and shipping tests. Below is the recognized consensus standards listed on the FDA website for this device:
- 3-96 ISO 81060-1 First edition 2007-12-01 Non-invasive sphygmomanometers - Part 1: Requirements and test methods for non-automated measurement type.
Manufacturing Intent
The device does not employ any area of manufacturing technology that is not well established.
Custom Reports

.png?width=4200&height=441&name=Zewski_Report%20Logo%20(Shirts).png)