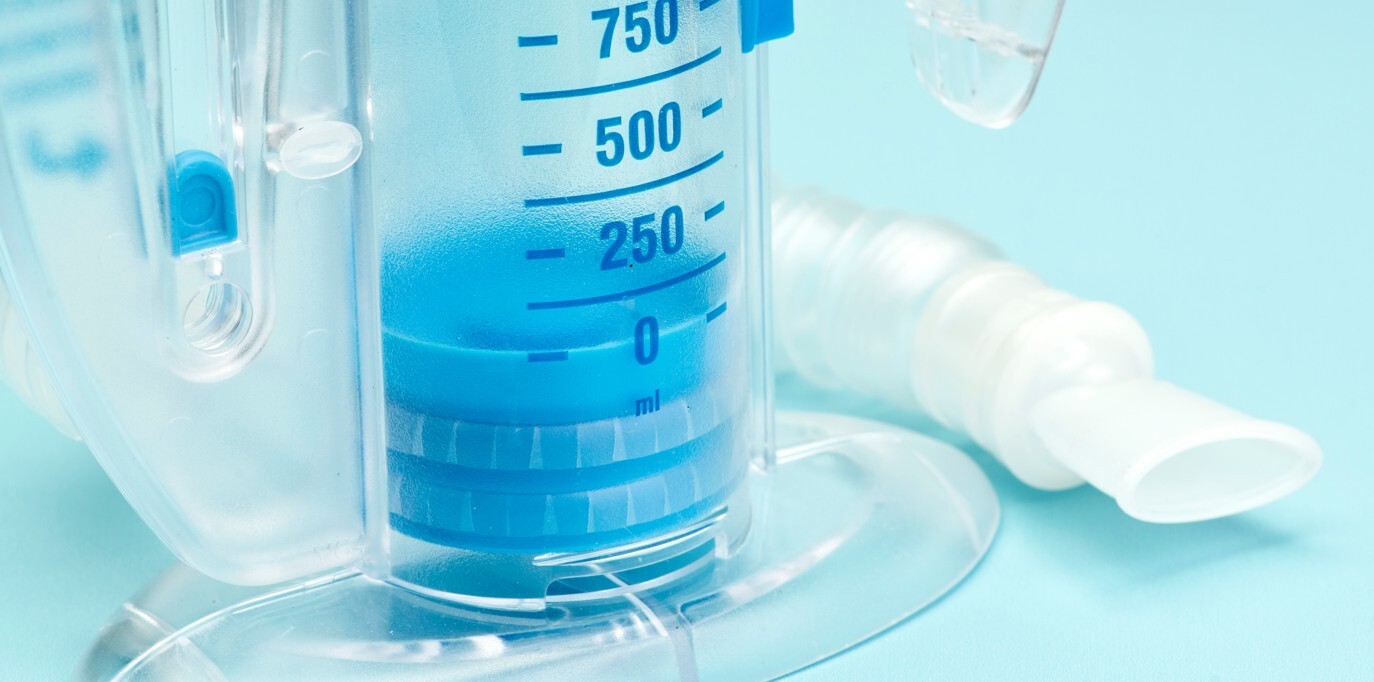
Incentive Spirometer, Or Similar
ABOUT THIS REPORT
Although this report focuses on the development of an incentive spirometer, the insights and methodology are broadly relevant to a wide range of similar medical devices providing general principles and realistic planning assumptions to guide innovators through the development landscape—especially for devices that might appear simple but involve hidden complexities.
All prebuilt Zewski Reports are developed with two core assumptions:
-
The product exists only as an idea.
Each report assumes the device is in its earliest conceptual stage and requires full development—covering human factor engineering, industrial design, mechanical and electrical engineering, prototyping, proto-production tooling, testing, regulatory clearance, and manufacturing planning. -
The device does not currently exist.
Even if the device appears simple or is already on the market in some form, our reports consider the effort required to create it as if it were a brand-new invention. They ask: What if this concept were being introduced for the first time? What technical, regulatory, and market challenges would need to be overcome? What resources would it take to bring a novel, ground-breaking version of this idea to life?
Zewski Reports do not account for "Me Too" products.
Our reports are not designed for estimating cost and time to duplicate existing solutions, which may require considerably less time and financial resources. Instead, they focus on the process and challenges of original innovation in today's market. We believe this give innovator the best glimpse of challenges in creating something groundbreaking.
DEVICE OVERVIEW
FDA Identification
An incentive spirometer is a device that indicates a patient's breathing volume or flow and that provides an incentive to the patient to improve his or her ventilation.
General Description
The incentive spirometer is a small, handheld respiratory therapy device designed to encourage deep breathing in patients recovering from surgery, illness, or extended periods of immobility. It operates through simple mechanical means, typically including a plastic chamber, a piston or ball to visually indicate airflow, and a flexible tube through which the patient inhales.
Unlike diagnostic spirometers, which measure lung function quantitatively, incentive spirometers provide visual feedback to motivate patients to achieve therapeutic breathing goals. The device does not contain any electronics or software components and requires no external power. Its simplicity and reusability make it an effective, low-cost solution in both hospital and home care settings.
This specific device concept remains in the early stages of development, with a focus on basic functionality rather than digital integration or customization. Materials are standard medical-grade plastics, and the design is reusable with minimal cleaning — a consideration that impacts both usability and regulatory pathways.
FEASIBILITY
Understanding Your Feasibility Score
The Feasibility Score bar provides an assessment of your project’s path to market, with higher values indicating lower complexity and fewer anticipated obstacles.
- 0 - 39 (Low Feasibility): This range suggests that the project may face significant challenges due to high complexity or extensive requirements. Additional planning, resources, or risk mitigation strategies will be necessary.
- 40 - 74 (Moderate Feasibility): Projects within this range indicate a moderate path to market. While the overall complexity is manageable, some areas may require refinement or further development to ensure project stability and success.
- 75+ (High Feasibility): A score in this range indicates a relatively straightforward path to market, with low complexity and minimal additional work expected. This project is well-positioned to progress smoothly.
The Feasibility Score is a general guide, not an absolute measure of project success. We recommend using this score as part of a broader assessment and considering additional expert guidance for a comprehensive evaluation.
PROJECT OVERVIEW
Note: This report incorporates certain assumptions based on our understanding of typical product development pathways and the stages at which our clients commonly engage with us. Where specific project details were unavailable, we’ve provided informed projections to support strategic planning.
This project centers on the development of a simple, mechanical incentive spirometer intended for therapeutic use. While the concept builds on a long-established product category, the project is still in its conceptual phase, without prior iterations, formal documentation, or institutional support. This early stage presents both challenges and opportunities — particularly in defining the product’s niche and planning a cost-effective development path.
Development Journey: Just Getting Started
The project is at the very beginning of the development journey — currently an idea or proof-of-concept with no formal prototyping or engineering work completed. The lack of design for manufacturing (DFM) considerations or testing history underscores that this is a pre-development initiative, and significant groundwork is needed before engaging with regulatory, production, or clinical stakeholders.
The absence of a clinical champion or institutional support means the project will need to generate credibility through strong prototyping, validation testing, and potential pilot trials. Strategic networking with clinicians, respiratory therapists, and procurement decision-makers will be critical as the concept advances.
The Competitive Context
Although the device itself is not functionally unique, this doesn't eliminate its potential. Instead, the lack of uniqueness shifts the focus to incremental innovation — such as improving ergonomics, simplifying the user experience, reducing manufacturing cost, or addressing sustainability in reuse and materials. Differentiation may also come from aligning with institutional purchasing trends (e.g., single-patient reusable devices, infection control priorities, or remote monitoring integration down the line).
What Lies Ahead
The next key steps involve defining clear product requirements, exploring cost-effective manufacturing methods, and confirming clinical usability. These tasks will be critical to refining the idea into a viable prototype that addresses real-world needs. Given the moderate supply chain complexity and some custom components, early supplier identification and design-for-assembly considerations will help reduce delays later in the process.
Strategic Takeaway:
Though still conceptual and not yet unique in function, this incentive spirometer project has a clear therapeutic purpose and a manageable development scope. Future success will depend less on innovation in core technology and more on clever execution, thoughtful design refinement, and early alignment with user needs and supply chain realities.
COST & TIME ESTIMATES
DEVELOPMENT COMPLEXITY
While the incentive spirometer is inherently a low-tech, mechanical device, certain factors still influence the complexity of its development. Understanding what simplifies the path forward — and what introduces cost or risk — will help the project team prioritize effectively and avoid unnecessary delays or rework.
Primary Drivers of Complexity
-
Custom Mechanical Components: Although the device is simple in concept, the presence of custom plastic parts and tubing assemblies may require early engagement with mold design and prototyping firms. Even minor variations in tolerance or assembly design can impact performance, especially for airflow-dependent indicators like pistons or float chambers.
-
Therapeutic Performance Validation: Because this is a therapeutic device, performance must be demonstrably reliable and consistent. Even if regulatory requirements are relatively light, verification and validation testing will be essential to prove the device achieves the intended clinical benefit.
-
Supply Chain Coordination: With moderate supply chain complexity and some custom parts, sourcing and logistics will require upfront planning. Missteps here could delay prototype builds or create cost overruns during production scaling.
What Simplifies Development
-
No Electronics or Software: The absence of sensors, firmware, or connectivity eliminates entire categories of risk, testing, and certification — including electrical safety, cybersecurity, and software validation.
-
No Power Source: As a manually powered device, the spirometer avoids dependence on batteries or power regulation components, further simplifying design and compliance.
-
Skin Contact Only: The device only contacts external parts of the body, minimizing biocompatibility concerns. This avoids more complex biological testing typically required for implantable or mucosal-contact devices.
What Introduces Complexity (and Cost)
-
Reusable Design: While reusability offers cost savings for the end user, it introduces regulatory and design implications — namely, cleanability and material durability. Even “minimal cleaning” claims must be supported by appropriate testing.
-
Patent Landscape: With several existing patents in the space, design choices must be made carefully to avoid infringement. Even with a “patent pending” status, freedom-to-operate analysis is recommended during design refinement.
-
Lack of Clinical Input: Without a clinical advisor or institutional partner, the project may struggle to validate whether the design truly fits user needs, leading to potential rework in later phases.
Strategic Takeaway:
Though mechanically simple, this device’s development is not without its challenges. The combination of custom mechanical design, reusable use-case, and limited clinical feedback creates a need for careful early planning. Streamlining complexity starts with solid prototyping and clear product definition rooted in user and supply chain realities.
TECHNOLOGICAL READINESS
Note: This report incorporates certain assumptions based on our understanding of typical product development pathways and the stages at which our clients commonly engage with us. Where specific project details were unavailable, we’ve provided informed projections to support strategic planning.
The incentive spirometer project is still in the concept phase, without physical prototypes, technical documentation, or prior iterations. At this stage, the focus should shift toward defining the device’s specifications, validating key performance criteria, and building an initial prototype that can evolve through iterative development.
Current Stage of Development
-
Concept Only: The device exists as a concept or proof-of-concept, without engineering design files, test data, or physical models.
-
No Iterative Refinement Yet: There have been no prior design iterations, which suggests the team has not yet tested user interaction, airflow behavior, or manufacturing feasibility.
-
No Documentation: With no technical documentation in place, the project is currently vulnerable to delays once external partners (e.g., manufacturers, regulatory consultants) become involved. Even simple devices benefit from early creation of design inputs, risk analyses, and user requirements.
Existing Technical Assets
-
Patent Pending Status: The project holds a pending patent, which indicates some level of technical definition. However, because the device does not yet have unique features or performance benchmarks, this pending status may be more conceptual than defensible. With several existing patents already in the market, a freedom-to-operate (FTO) review will be essential before moving into design finalization.
-
No Design for Manufacturing (DFM) Consideration: Manufacturing feasibility has not yet been assessed. As the design evolves, incorporating DFM principles will help reduce cost, simplify assembly, and minimize errors during scale-up.
What Comes Next
To transition from idea to functioning prototype, the next steps should include:
-
Establishing Design Inputs: Define the intended use, user needs, performance criteria, and environmental conditions. This step is foundational to engineering design and regulatory planning.
-
Prototyping with User Feedback: Rapid prototyping of the airflow mechanics and user interface (mouthpiece, visual indicator, tubing) should occur early, ideally with feedback from clinicians or therapists.
-
Early Performance Benchmarks: Even without regulatory submissions, basic airflow validation or usability evaluations can guide refinement and reduce later-stage risk.
-
Initial Design Documentation: Begin compiling core documents — such as block diagrams, user needs, and risk considerations — that will eventually support verification and regulatory review.
Strategic Takeaway:
The project has a solid conceptual foundation but is at zero technical maturity in practical terms. Investing now in basic prototyping, design inputs, and performance validation will accelerate the project’s ability to secure funding, partners, and clinical interest while laying the groundwork for formal development.
REGULATORY APPROVAL
The incentive spirometer is classified as a Class II medical device in the U.S. and typically requires a 510(k) premarket notification for regulatory clearance. While this is a well-established regulatory pathway with clear precedents, there are still key activities and documentation requirements that must be addressed — even for a device without electronics or complex components.
The FDA evaluates incentive spirometers based on substantial equivalence to legally marketed predicate devices. Most products in this category demonstrate safety and effectiveness through bench testing and usability validation, rather than clinical trials. However, given the device’s reusable nature, cleanability, material safety, and labeling claims will receive close scrutiny.
FDA Classification Snapshot
- Regulation Number: 868.5690
- Product Code: BWF
- Regulation Medical Specialty: Anesthesiology
- Device Class: Class II
- Submission Pathway: 510(k) Premarket Notification
You should work with a regulatory consultant to verify the correct classification and any associated guidance documents.
Predicate Comparison
To complete a 510(k) submission, the product must be shown to be substantially equivalent to a legally marketed predicate. The device’s lack of unique features may make it easier to align with existing products — but it's still essential to define and document similarities and differences in:
- Intended use
- Technological characteristics (e.g., airflow mechanism)
- Performance specifications
Biocompatibility Testing
Although the spirometer has skin contact only, materials used in mouthpieces or tubing must be evaluated for biocompatibility per ISO 10993 standards. Testing requirements may include:
- Cytotoxicity
- Sensitization
- Irritation
Material documentation and historical safety data may be sufficient for well-characterized plastics, but testing is often still recommended to ensure compliance.
Reuse and Cleaning Validation
The spirometer is described as reusable with minimal cleaning, which places additional responsibility on the manufacturer to:
- Define the cleaning process clearly in labeling
- Validate that the process effectively removes contaminants without degrading device performance or materials
This is a common area of scrutiny in FDA reviews, especially if reuse could introduce infection risk.
Labeling Requirements
Labeling must clearly reflect:
- Intended use
- Cleaning instructions
- Warnings and precautions
- Performance claims
Because users may include non-clinical caregivers, readability and proper instruction format (e.g., pictograms) can improve both safety and compliance.
International Considerations (Optional)
If international markets are of interest, CE marking under the EU MDR would require conformity assessment under Class IIa, including a technical file, risk management report, and post-market surveillance planning. Harmonized standards for biocompatibility, usability, and labeling are aligned with FDA expectations in many areas, but timelines and documentation formats differ.
Strategic Takeaway:
While the 510(k) path is well-trodden for incentive spirometers, it still demands careful documentation, robust predicate analysis, and validation of safety claims — especially for reusable devices. Early regulatory planning and alignment with FDA expectations can reduce delays and help ensure a smooth submission process.
MARKET POTENTIAL
The market for incentive spirometers is mature, but steady — driven by widespread clinical protocols that require post-operative respiratory therapy and by institutional priorities around preventing pulmonary complications. While the device concept itself is not novel, there remains meaningful opportunity in cost efficiency, usability, and alignment with healthcare system priorities.
Market Drivers
- Post-Surgical and Bedside Use: Hospitals routinely provide incentive spirometers to patients after abdominal or thoracic surgeries to prevent atelectasis. This creates a consistent demand pipeline, especially in acute care and rehabilitation settings.
- Chronic Respiratory Management: In some cases, incentive spirometers are used in outpatient or home care for conditions like COPD or pneumonia recovery, expanding their relevance to long-term care and respiratory therapy programs.
- Clinical Protocol Integration: The inclusion of spirometry in enhanced recovery protocols, infection prevention strategies, and discharge bundles ensures ongoing demand within institutional settings.
Target Segments
- Hospitals and Surgical Centers: The largest and most consistent buyers, typically through bulk purchasing agreements or procurement contracts.
- Home Health Providers and Pharmacies: Serve patients recovering at home; potential for direct-to-consumer offerings.
- Rehabilitation Facilities and Long-Term Care: Offer continuity of care and could value improved reusability or patient engagement features.
Adoption Enablers
- Low Training Requirement: The simplicity of the device allows rapid adoption without significant onboarding.
- Familiarity in Clinical Practice: Widespread use in hospitals means minimal clinical resistance — assuming design changes do not compromise usability.
- Cost Sensitivity of Buyers: Because the device is low-cost and often bundled with care, procurement decisions are driven by unit price, reusability, and logistical simplicity.
Revenue Considerations
- Per-Unit Margins Are Tight: High-volume manufacturing and cost control are essential, especially when competing in price-sensitive institutional markets.
- Private Labeling or Licensing: There may be opportunities to partner with existing distributors or manufacturers looking to extend their product line with branded variations.
Revenue Risk Factors
- Commoditization: With many similar products on the market, pricing pressure is high unless a meaningful differentiator (e.g., better ergonomics, sustainability, infection control features) is introduced.
- Procurement Barriers: Without clinical advocacy or institutional support, breaking into hospital supply chains can be difficult.
- Regulatory Delays: While the 510(k) path is straightforward, failure to meet testing or labeling requirements can delay market entry and strain early-stage resources.
Strategic Takeaway:
This device operates in a stable but competitive market, where differentiation must come from value-driven innovation — not revolutionary features. A thoughtful design, a strong manufacturing plan, and alignment with institutional needs are key to achieving commercial traction and long-term sustainability.
DEVELOPMENT PHASES & MILESTONES
To bring the incentive spirometer from concept to market, the project should follow a structured, milestone-driven development path. Although the device is mechanically simple, each phase serves a critical purpose in ensuring the design is clinically effective, manufacturable, and regulatory compliant. Below are the key phases tailored to this device's profile.
Phase I: Concept Development
Goal: Translate the initial idea into defined product requirements and prepare for prototyping.
Key Activities:
- Define user needs and clinical context.
- Research predicate devices and identify key differentiators.
- Develop preliminary design inputs (performance specs, user interface, intended use).
- Evaluate freedom-to-operate based on existing patents.
Milestone: Documented product requirements and conceptual design sketch or model.
Phase II: Prototype Development
Goal: Build and refine a working prototype based on defined specifications.
Key Activities:
- Design airflow mechanism and housing components in CAD.
- Select materials for tubing, indicators, and housing.
- 3D print or fabricate prototype parts for initial testing.
- Conduct informal usability tests with target users (e.g., therapists).
Milestone: Functional prototype ready for performance and usability testing.
Note: The regulatory cost estimates in this section include expenses associated with an optional FDA 510(k) pre-submission (Q-Sub), which, while not required, can be a valuable tool for obtaining early feedback and reducing downstream submission risk.
Phase III: Design Output & Verification
Goal: Finalize the design and verify that it meets all defined requirements.
Key Activities:
- Iterate design based on prototype feedback.
- Perform performance tests (e.g., airflow resistance, repeatability).
- Develop cleaning instructions and assess material durability.
- Prepare design history file (DHF) components.
Milestone: Verified design ready for validation and regulatory documentation.
Performance Testing Matrix
| Test Name | Standard / Reference | Purpose |
| Flow Resistance Testing | ISO 5367:2015 (Respiratory therapy equipment) | Ensure that airflow resistance is within acceptable limits for therapeutic effectiveness. |
| Inspiratory Volume Testing | Internal Standard or FDA Guidance | Validate that the device accurately measures or provides feedback on the volume of air inspired. |
| Mechanical Durability | Internal Test Protocols | Simulate long-term usage to ensure that mechanical parts (e.g., pistons, springs) can withstand multiple uses without failure. |
| Feedback Indicator Accuracy | ISO 5367:2015 | Verify the accuracy of the feedback mechanism (e.g., visual indicator or volume gauge). |
Biological Safety Testing Matrix
| Test Name | Standard / Reference | Purpose |
| Biocompatibility Testing | ISO 18562 | Assess materials that come into contact with skin or mucous membranes to ensure they do not cause adverse reactions. |
| Cleaning & Sterilization Validation | Internal Protocols or FDA Guidelines | Validate that the device can be effectively cleaned and disinfected between uses to prevent cross-contamination. |
* The tests above are provided as illustrative examples to reflect the expected level of complexity and rigor required during the development of the product. Final tests, plans and protocols may vary based on the finalized design, risk assessment, and regulatory strategy.
Phase IV: Validation & Regulatory Submission
Goal: Validate the device in real-world conditions and submit a 510(k) premarket notification.
Key Activities:
- Conduct usability validation in a simulated or clinical setting.
- Complete biocompatibility and cleaning validation testing.
- Prepare labeling, instructions for use, and 510(k) documentation.
- Identify and document predicate comparison.
Milestone: 510(k) submission filed with the FDA.
Usability & Labeling Testing Matrix
| Test Name | Standard / Reference | Purpose |
| Usability (Human Factors) Validation | FDA Human Factors Guidance / IEC 62366-1 | Demonstrate safe and intuitive operation across users/settings |
| Label Durability Testing | ISO 60601-1 + Internal Protocol | Ensure labels stay readable during use/exposure to moisture or cleaning |
Packaging and Environmental Testing Matrix
| Test Name | Standard / Reference | Purpose |
| Packaging Integrity Testing | ISTA/ASTM D4169 | Ensure that packaging protects the device during transport and storage. |
| Environmental Durability Testing | ISO 13485 | Simulate environmental conditions (e.g., temperature, humidity) to ensure the device's integrity throughout the supply chain |
Phase V: Full-Scale Production & Launch
Goal: Scale manufacturing and introduce the product to the market.
Key Activities:
- Finalize supply chain and production tooling (e.g., injection molds).
- Implement quality control and post-market surveillance plan.
- Train distributors or sales teams.
- Launch marketing campaign targeting institutions and home health providers.
Milestone: Commercial launch with initial sales and manufacturing scalability in place.
Each phase has its own technical and business challenges — but the biggest delays typically happen when design, testing, or regulatory planning are rushed or skipped early on. By following a phased model and closing out each milestone thoroughly, you set yourself up for a smoother regulatory path, stronger manufacturing handoff, and faster market entry.
RESOURCE ALLOCATION & TEAM INVOLVEMENT
Although the incentive spirometer is a mechanically simple device, bringing it to market still requires coordination across multiple disciplines. Because this project is at the concept stage, resource planning should focus on building a lean, versatile team that can execute key activities efficiently — particularly in prototyping, testing, and regulatory documentation.
Core Functional Roles Required
- Product Development Engineer: Leads design, CAD modeling, and prototyping. Also coordinates performance verification.
- Regulatory Consultant: Advises on 510(k) submission content, predicate comparison, and biocompatibility requirements.
- Industrial Designer (Optional): Can improve form factor and usability, especially if differentiating through ergonomics or patient engagement.
- Manufacturing Engineer: Assesses design for manufacturability (DFM), selects materials, and supports scale-up planning.
Specialty Support Needs
- Intellectual Property Attorney: Supports ongoing patent prosecution, performs FTO analysis, and advises on competitive IP positioning.
- Clinical Advisor (e.g., Respiratory Therapist): Provides feedback on usability, cleaning, and patient compliance — especially valuable in prototype testing and labeling development.
- Quality Assurance Specialist: Assists with documentation of design controls and preparation for regulatory submission.
- Supply Chain Manager or Consultant: Sources components, identifies production partners, and ensures cost-effective material planning.
| Phase | Contributors |
| Concept | Inventor, Clinical Advisor, IP Attorney |
| Prototype | Product Engineer, Industrial Designer |
| Testing & Validation | Engineer, Clinical Advisor, QA Specialist |
| FDA Submission | Regulatory Consultant, QA Specialist |
| Production & Launch | Manufacturing Engineer, Supply Chain Lead, Marketer |
Note: One individual may serve multiple roles, especially in lean startup environments.
Strategic Takeaway:
Even for simple devices, cross-functional expertise is essential. Building the right team early — especially with strong engineering and regulatory guidance — lays the foundation for faster development, better documentation, and a smoother regulatory path.
RISK MITIGATION STRATEGIES
Risk management for an incentive spirometer may appear straightforward due to the device’s mechanical simplicity and lack of electronics. However, regulatory expectations still require a thorough assessment of usability, performance, material safety, and manufacturing reliability — particularly since the device is reusable and patient-operated.
Usability Risks
Key Risks:
- Improper patient use due to misunderstanding instructions
- Insufficient inspiratory effort, leading to ineffective therapy
- Device orientation or positioning errors that affect feedback indicators
Mitigation Strategies:
- Include simple, well-illustrated instructions for use (IFU)
- Conduct usability studies with lay users and clinical staff
- Incorporate design features that guide correct hand placement and posture
Performance Risks
Key Risks:
- Inaccurate or inconsistent airflow resistance or volume feedback
- Mechanical failure or blockage in airflow pathway
- Inadequate durability over repeated use
Mitigation Strategies:
- Perform bench testing to validate performance tolerances
- Select materials and spring mechanisms with proven stability
- Define clear performance acceptance criteria during verification testing
Electrical/Mechanical Safety Risks
Key Risks:
- Minimal in this case; the device contains no electronics
- Mechanical components (e.g., moving indicator, piston) may stick or degrade over time
Mitigation Strategies:
- Use wear-resistant materials for all moving parts
- Simulate repeated use to evaluate long-term mechanical reliability
Regulatory Risks
Key Risks:
- Incomplete or insufficient 510(k) documentation
- Failure to validate reusability or biocompatibility
- Misalignment with predicate devices or labeling standards
Mitigation Strategies:
- Engage a regulatory consultant early in the design process
- Establish traceable design controls and documentation from the start
- Conduct a thorough predicate analysis and risk assessment
Manufacturing and Supply Chain Risks
Key Risks:
- Variability in plastic component quality or tolerances
- Long lead times for custom tubing or indicator components
- Assembly challenges if components require tight tolerances
Mitigation Strategies:
- Source manufacturers with medical device experience
- Design for manufacturability (DFM) early to avoid rework
- Qualify backup suppliers for critical custom parts
Strategic Takeaway:
For a Class II mechanical device, risk management is less about complexity and more about consistency. Early, structured testing and thoughtful design documentation help prevent delays and ensure regulatory alignment — especially for reusable devices expected to perform reliably across multiple uses.
INVESTMENT & FINANCIAL OUTLOOK
While the incentive spirometer is relatively inexpensive to produce and has a straightforward development path, financial success depends on cost efficiency, clear value proposition, and volume-based strategies. Early inventors must approach budgeting and fundraising with a focus on lean execution and smart positioning in a commoditized market.
Primary Cost Drivers
- Tooling and Manufacturing Setup: Injection molding and assembly line tooling can be expensive upfront, especially for multi-component designs with tight tolerances.
- Regulatory Testing and Submission: Though the 510(k) path is less burdensome than PMA, costs related to performance testing, biocompatibility validation, and consultant fees can accumulate quickly.
- Prototyping and Iteration: Several rounds of mechanical refinement may be needed to meet performance and durability benchmarks — especially if the device is designed for reuse.
- Legal and IP Expenses: With a “patent pending” status and several existing patents in the space, strategic patent prosecution and FTO (freedom to operate) analyses are necessary to secure competitive ground.
Budgeting Tips for Early Inventors
- Focus on Proof of Function First: Demonstrate clear mechanical reliability and usability before investing in final production design or regulatory submission.
- Use Modular Prototyping: Design components that can be easily swapped or adjusted to reduce the cost of iterations.
- Leverage Grant and Institutional Support: While currently lacking, clinical partnerships could unlock access to non-dilutive funding or pilot sites.
Funding Strategy Considerations
- Angel Investors or Seed Capital: Useful for covering early design and prototyping costs, particularly if IP has early-stage value.
- Strategic Partnerships: Aligning with a distributor or contract manufacturer may offer both capital and faster market access.
- Crowdfunding or Direct-to-Consumer Pilots: In niche outpatient applications, this could validate demand and generate early revenue data.
Revenue Potential Considerations
- High Volume, Low Margin Model: The typical business model depends on scale and operational efficiency, especially in hospital or institutional markets.
- Private Label Opportunities: Some manufacturers may license or rebrand a new spirometer design with minor adjustments, offering a non-traditional path to commercialization.
- Sustainability Differentiation: Emphasizing reusable, recyclable, or more hygienic features could create small but meaningful pricing leverage.
Financial Risk Mitigation
- Stage-Gated Spending: Align spending with product maturity — defer high-cost items like mold fabrication until after regulatory testing is passed.
- Outsource Strategically: Contract specialists (e.g., regulatory, IP, QA) only when needed to avoid ballooning fixed costs.
- Plan for Commodity Pressure: Keep BOM (bill of materials) costs low and validate pricing scenarios early based on existing market benchmarks.
Strategic Takeaway:
Success in the incentive spirometer market depends less on novelty and more on smart execution. Financial discipline, early clarity in the value proposition, and strategic partnerships can position even a simple device for sustainable revenue within a mature market.
Understanding Vendor Tiers and Impact on Project Cost and Time
Tier 1: Higher costs associated with comprehensive services complete system development, advanced technology, and the ability to manage complex projects. Design services may have shorter lead times due to ability to build a larger team however the scale of operations and the complexity of the more comprehensive supply chain may slow certain processes.
Tier 2: Their cost and Time may vary based on their specialization allowing for efficient production of specific components, potentially leading to shorter lead times for those items. However, since they do not provide complete systems, the overall integration into larger assemblies may require additional coordination, potentially affecting timelines.
Tier 3: Lower costs due to specialization in specific components or materials or limited staffing resources requiring additional coordination with other suppliers. This may slow the development time from both a design and supply chain perspective.
Considerations
- Despite higher costs and longer lead times, Tier 1 suppliers may be more suitable for complex projects requiring integrated solutions.
- For projects with budget constraints, engaging multiple Tier 3 suppliers could be more cost-effective, but may require more intensive project management.
- Working with Tier 3 suppliers entails coordinating a robust supply chain to ensure timely delivery and quality assurance.
The choice between Tier 1 and Tier 3 suppliers involves trade-offs between cost, time, and supply chain management complexity. Careful evaluation of project requirements and resources is essential for making an informed decision.
Disclaimers & Limitations
- Generalizations: This report provides a high-level overview based on standard assumptions and does not account for unique device characteristics. Actual costs, timelines, and risks may vary significantly depending on the device's design, use case, and target market.
- Assumptions of Device Class and Use: Assumptions were made regarding the device's classification and intended use. These assumptions can impact regulatory requirements, costs, and timelines. Specific regulatory pathways, for instance, may differ based on the device's risk classification and market entry strategy.
- Market and Regulatory Dynamics: Regulatory requirements and market conditions are subject to change. The report's cost and timeline estimates may be affected by evolving regulatory landscapes, standards, or unforeseen market dynamics, which could delay approval or require additional testing.
- Risk Assessment Limitations: Risk levels and mitigation strategies are based on general device categories and may not fully address specific technical or operational risks unique to the product. Thorough risk assessments should be tailored to the device's complexity, materials, and usage.
- Development Phases and Milestones: The development phases outlined here follow a typical medical device development pathway, but real-world project phases may overlap or require iteration due to unforeseen challenges or design changes.
- Cost and Timeline Variability: The cost and timeline estimates are based on standard industry benchmarks but do not account for project-specific adjustments. Factors like unexpected technical challenges, prototype iterations, or regulatory re-submissions can significantly impact final costs and schedules.
- Reliance on Industry Standards: The report relies on common industry standards for development and testing. However, additional standards specific to certain device features or regions may apply, affecting compliance requirements and associated timelines.
- Testing and Validation Scope: Testing and validation requirements are generalized. Devices with novel materials, complex electronics, or unique features may require additional, specialized tests, potentially extending both cost and duration.
.png?width=4200&height=441&name=Zewski_Report%20Logo%20(Shirts).png)