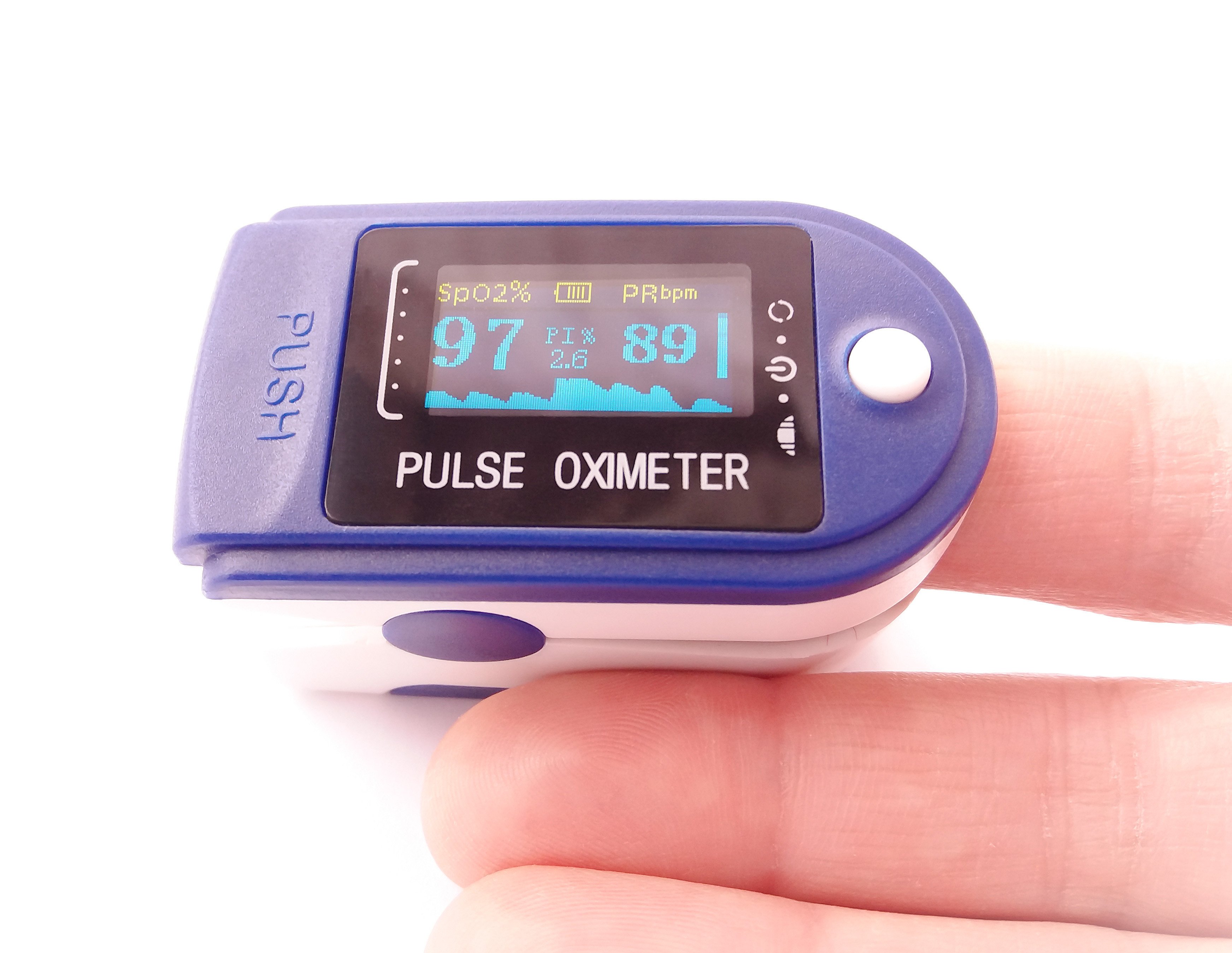
Pulse Oximeter
Device Type: Cardiovascular - Monitoring
FDA Description:
A pulse oximeter is a Class II medical device that non-invasively measures blood oxygen saturation (SpO₂) and pulse rate. It uses light absorption through a finger, earlobe, or other tissue to provide real-time readings. The FDA regulates pulse oximeters under strict safety and performance standards.
General Description:
A pulse oximeter is a compact, non-invasive medical device that measures a patient’s blood oxygen saturation (SpO₂) and pulse rate. Widely used in clinical and home settings, it provides real-time monitoring of oxygen levels, critical for patients with respiratory conditions, cardiovascular issues, or during surgical procedures.
The device operates by emitting light through a translucent body part, such as a fingertip or earlobe. It detects changes in light absorption by oxygenated and deoxygenated hemoglobin, calculating the percentage of oxygen saturation in the blood. Modern pulse oximeters feature digital displays, providing clear and immediate results.
Pulse oximeters are available in various forms, including handheld, tabletop, and fingertip models, offering portability and convenience. Advanced models may include alarms for abnormal readings, wireless connectivity for data sharing, and integration with patient monitoring systems.
These devices are essential in hospitals, emergency care, and home settings, especially for individuals with conditions such as COPD, asthma, or COVID-19. Pulse oximeters meet stringent FDA requirements to ensure accuracy, reliability, and safety. By providing continuous, non-invasive monitoring, they empower healthcare providers and patients to manage health conditions effectively and respond promptly to critical changes.
Pulse Oximeter
General Report
Project Overview
Cost & Time Estimates
Development Complexity
Technological Readiness
Regulatory Approval
Market Potential
Development Phases & Milestones
Resource Allocation & Team Involvement
Risk Mitigation Strategies
Investment & Financial Outlook
Feasibility
Understanding Your Feasibility Score
The Feasibility Score bar provides an assessment of your project’s path to market, with higher values indicating lower complexity and fewer anticipated obstacles.
- 0 - 39 (Low Feasibility): This range suggests that the project may face significant challenges due to high complexity or extensive requirements. Additional planning, resources, or risk mitigation strategies will be necessary.
- 40 - 74 (Moderate Feasibility): Projects within this range indicate a moderate path to market. While the overall complexity is manageable, some areas may require refinement or further development to ensure project stability and success.
- 75+ (High Feasibility): A score in this range indicates a relatively straightforward path to market, with low complexity and minimal additional work expected. This project is well-positioned to progress smoothly.
The Feasibility Score is a general guide, not an absolute measure of project success. We recommend using this score as part of a broader assessment and considering additional expert guidance for a comprehensive evaluation.
Cost & Time Breakdown
Phase I
Phase II
Phase III
Phase IV
Phase V
Disclaimers & Limitations
- Generalizations: This report provides a high-level overview based on standard assumptions and does not account for unique device characteristics. Actual costs, timelines, and risks may vary significantly depending on the device's design, use case, and target market.
- Assumptions of Device Class and Use: Assumptions were made regarding the device's classification and intended use. These assumptions can impact regulatory requirements, costs, and timelines. Specific regulatory pathways, for instance, may differ based on the device's risk classification and market entry strategy.
- Market and Regulatory Dynamics: Regulatory requirements and market conditions are subject to change. The report's cost and timeline estimates may be affected by evolving regulatory landscapes, standards, or unforeseen market dynamics, which could delay approval or require additional testing.
- Risk Assessment Limitations: Risk levels and mitigation strategies are based on general device categories and may not fully address specific technical or operational risks unique to the product. Thorough risk assessments should be tailored to the device's complexity, materials, and usage.
- Development Phases and Milestones: The development phases outlined here follow a typical medical device development pathway, but real-world project phases may overlap or require iteration due to unforeseen challenges or design changes.
- Cost and Timeline Variability: The cost and timeline estimates are based on standard industry benchmarks but do not account for project-specific adjustments. Factors like unexpected technical challenges, prototype iterations, or regulatory re-submissions can significantly impact final costs and schedules.
- Reliance on Industry Standards: The report relies on common industry standards for development and testing. However, additional standards specific to certain device features or regions may apply, affecting compliance requirements and associated timelines.
- Testing and Validation Scope: Testing and validation requirements are generalized. Devices with novel materials, complex electronics, or unique features may require additional, specialized tests, potentially extending both cost and duration.
- Supplier Chain for Complex Equipment: If your device includes advanced 'add-in' equipment like sensors, lasers, pumps, or vision systems, etc., the development timeline and associated costs will require a custom report.
.png?width=4200&height=441&name=Zewski_Report%20Logo%20(Shirts).png)